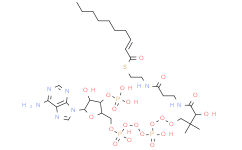
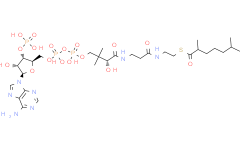
产品名称: Caproyl-CoA
其他名称: 己酰辅酶A;Hexanoyl-Coenzyme A
CAS:5060-32-2
其他名称: 己酰辅酶A;Hexanoyl-Coenzyme A
CAS:5060-32-2
结构信息
| 分子式 | C27H46N7O17P3S | 分子量 | 865.68 | ||
| IUPAC Name | S-[2-[3-[[(2R)-4-[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethyl] hexanethioate | ||||
| InChIKey | OEXFMSFODMQEPE-HDRQGHTBSA-N | ||||
| InChI | InChI=1S/C27H46N7O17P3S/c1-4-5-6-7-18(36)55-11-10-29-17(35)8-9-30-25(39)22(38)27(2,3)13-48-54(45,46)51-53(43,44)47-12-16-21(50-52(40,41)42)20(37)26(49-16)34-15-33-19-23(28)31-14-32-24(19)34/h14-16,20-22,26,37-38H,4-13H2,1-3H3,(H,29,35)(H,30,39)(H,43,44)(H,45,46)(H2,28,31,32)(H2,40,41,42)/t16-,20-,21-,22+,26-/m1/s1 | ||||
| SMILES | CCCCCC(=O)SCCNC(=O)CCNC(=O)C(C(C)(C)COP(=O)(O)OP(=O)(O)OCC1C(C(C(O1)N2C=NC3=C(N=CN=C32)N)O)OP(=O)(O)O)O | ||||
相关文献