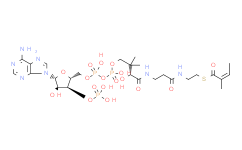
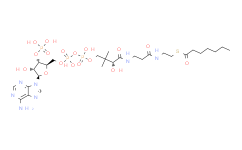
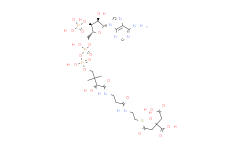
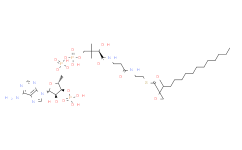
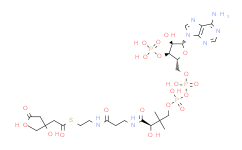
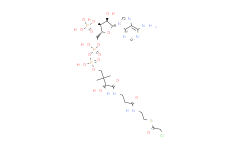
产品名称: Malonyl-CoA
其他名称: S-(hydrogen malonyl)coenzyme A;Malonyl coenzyme A;Coenzyme A, S-(hydrogen propanedioate)
CAS:524-14-1
其他名称: S-(hydrogen malonyl)coenzyme A;Malonyl coenzyme A;Coenzyme A, S-(hydrogen propanedioate)
CAS:524-14-1
结构信息
| 分子式 | C24H38N7O19P3S | 分子量 | 853.58 | ||
| IUPAC Name | S-[2-[3-[[4-[[[5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethyl] 3-hydroxy-2-methylbutanethioate | ||||
| InChIKey | PEKYNTFSOBAABV-UHFFFAOYSA-N | ||||
| InChI | InChI=1S/C26H44N7O18P3S/c1-13(14(2)34)25(39)55-8-7-28-16(35)5-6-29-23(38)20(37)26(3,4)10-48-54(45,46)51-53(43,44)47-9-15-19(50-52(40,41)42)18(36)24(49-15)33-12-32-17-21(27)30-11-31-22(17)33/h11-15,18-20,24,34,36-37H,5-10H2,1-4H3,(H,28,35)(H,29,38)(H,43,44)(H,45,46)(H2,27,30,31)(H2,40,41,42) | ||||
| SMILES | CC(C(C)O)C(=O)SCCNC(=O)CCNC(=O)C(C(C)(C)COP(=O)(O)OP(=O)(O)OCC1C(C(C(O1)N2C=NC3=C(N=CN=C32)N)O)OP(=O)(O)O)O | ||||
相关文献